
The article by Analog Devices features a key challenge for clinical-grade wearables designers on making high-quality HR and SpO2 measurements in a way that doesn’t place a heavy drain on the battery of the device. In this design solution, the author shows why the conventional approach to optical readings wastes power before presenting a sensor IC that uses a novel architecture to substantially reduce power consumption while making clinical-grade measurements.
Heart rate (HR) and blood oxygen saturation (SpO2) are quickly moving from the “desirable” to the “expected” phase in the list of features available in health and fitness wearables. A consequence of this transition, however, has been a reduction in the quality of readings caused by some sensor manufacturers making questionable claims about the accuracy of their products in the rush to meet the market demand for these features. While the accuracy of readings may not be critical in everyday wearables, the quality and integrity of measurements must be unquestionable in clinical-grade wearables.
Photoplethysmography (PPG)
HR and SpO2 are measured using an optical technique called photoplethysmography or PPG (Figure 1). A PPG signal is obtained by illuminating skin using a light-emitting diode (LED) and detecting changes in the intensity of light reflected from blood vessels below the surface (Figure 2) using a photodiode (PD) that generates a current proportional to the amount of received light.
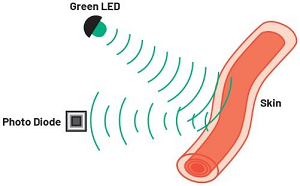
The current signal is conditioned by the PPG analog front end (AFE) before being converted by an ADC for processing by an optical algorithm running on the system’s microcontroller. In principle, a single LED-PD pair is sufficient to make a PPG measurement and this architecture is common in equipment used in clinical settings (Figure 3).

However, these devices operate in conditions very different to those encountered in everyday life. First, the patient is relatively immobile, and the measurement is performed using a sensor that is securely fastened to a fingertip. Lighting conditions are relatively constant, which simplify light detection for the PD and power consumption is not a concern since these devices are usually mains powered.
By contrast, a wearable device is typically wrist-worn, meaning the level of skin contact varies, depending on the personal preferences (strap tightness) and the motion of the wearer. Lighting conditions can vary considerably depending on location and time of day and since these devices are battery-powered, it is important for the current consumption of the sensor to be as low as possible. This is made even more challenging by the variety of skin tones of different wearers. Darker skin is described as having a lower perfusion index than lighter skin, meaning it requires greater illumination (requiring a sensor to use more power) for measurements to be made. We consider next the merits of different AFE architectures that can be used to make PPG measurements.
PPG AFE with Single ADC channel
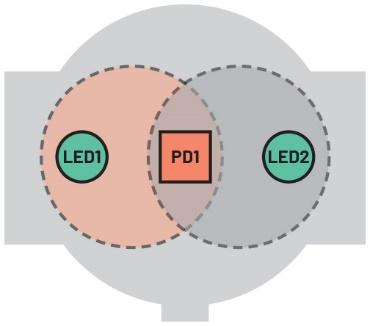
Increasing LED current, or using two LEDs, is an intuitive way to achieve a higher degree of skin illumination (Figure 4) since it can illuminate a greater skin area. However, this is a power-hungry approach because LED current accounts for at least 50% of the power consumed in a PPG system that, depending on the skin perfusion index of the wearer, can be up to an average of 1 mW. Overall, this approach is inefficient and detrimental to battery life.
PPG AFE with Dual ADC Channels
A better way to increase skin illumination is to use a single LED with two PDs that can be used to detect a greater amount of reflected light (Figure 5).
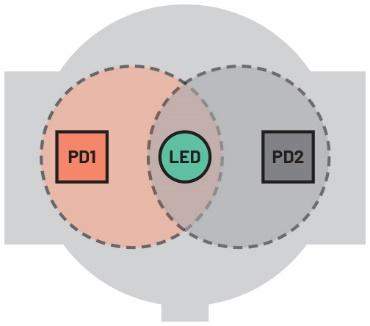
The advantage here is that the standard 20 mA LED current can be reduced to 10 mA to achieve the same level of total PD current when compared to using a single PD. In challenging operating conditions (low skin perfusion and/or when the wearer is moving) where the system algorithm determines that a higher LED current is required, a proportional increase in system sensitivity can be attained. For example, applying the same LED current as in the previous arrangement delivers a 100% increase in PD current, thus delivering higher overall sensitivity, albeit at the cost of increased power consumption.
PPG AFE with Quad ADC Channels
Using four PDs (which require a quad-channel ADC) to detect reflected light saves yet more power (Figure 6) as the LED can be run for a lot less power (Table 1).
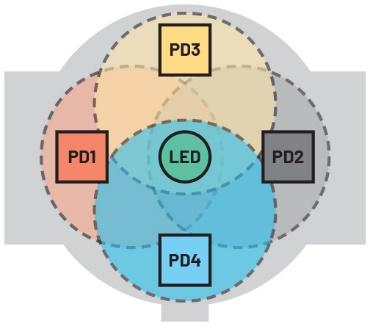
Table 1 summarizes the relative power consumption of each of the architectures previously considered, assuming a 1.6 V typical supply voltage.
Table 1. Comparison of the Typical Power Consumption for 1-, 2-, and 4-Channel ADC Architectures
| Number of PDADC Channels | No. ofLEDs | LED Current(mA) | LED Power(mW) | AFE Power(mW) | Total Power(mW) |
| 1 | 1 | 20 | 320 | 30 | 350 |
| 2 | 1 | 10 | 160 | 40 | 200 |
| 4 | 1 | 5 | 80 | 60 | 140 |
This architecture delivers higher quality readings because blood vessels and bones are distributed asymmetrically in the wrist and four PDs help mitigate the effects of motion and how tight the wearer fastens the device. Four PD receivers also increase the probability of detecting light reflected from illuminated blood vessels. The graph in Figure 7 shows HR measured using four photodiodes (configured as two independent pairs: LEDC1 and LEDC2) with respect to a reference measurement (polar). The wearable needs to ensure that a good skin contact is maintained while this measurement is being performed. Initially, the wearer is at rest, then after 5 minutes (300 seconds) they begin exercising causing their HR to increase. It is clear that the signals on LEDC1 and LEDC2 deviate differently from the reference measurement and the benefit of using two pairs of PDs to capture and combine all of these deviations is apparent.
Figure 7. HR readings using two pairs of independent PDs.
Figure 8. A MAX86177 quad-channel optical AFE block diagram.
Practical Quad ADC Solution
The MAX86177 (Figure 8) is an ultra low power quad-channel optical data acquisition system with both transmit and receive channels that is ideal for use in clinical-grade (as well as general purpose) portable and wearable devices. On the transmitter side, it has two high current 8-bit programmable LED drivers that support up to six LEDs. On the receiver side, it has four low noise charge integrating front ends that each include independent 20-bit ADCs that can multiplex input signals from eight PDs (configured as four independent pairs). It achieves a dynamic range of 118 dB and provides ambient light cancellation (ALC) up to 90 dB at 120 Hz. It operates on a 1.8 V main supply voltage with a 3.1 V to 5.5 V LED driver supply voltage. The device provides fully autonomous support for both I2C and SPI compatible interfaces. The MAX86177 is available in a 7 × 4, 28-ball wafer-level package (WLP) with dimensions of 2.83 mm × 1.89 mm and that operates over –40ºC to +85ºC temperature range. Lab-tested samples of this AFE exhibited an overall root-mean-square error for hypoxia measurement of 3.12%, well inside the 3.5% limit set by the FDA for clinical-grade monitors.
Conclusion
A major headache for designers of clinical-grade wearables is how to make optical PPG measurements of HR and SpO2 without placing a significant drain on the battery life of the device. In this design solution, we have shown that a four-channel ADC architecture can deliver power savings of up to 60% when compared to the basic architecture that uses a single LED and PD. The MAX86177’s four-channel architecture in a small form factor package is ideal for use in finger, wrist and ear-worn wearables to measure clinical-grade HR and SpO2. It can also be used to measure body hydration, muscle, and tissue oxygen saturation (SmO2 and StO2) and maximum oxygen consumption (VO2 Max).
About the Author
Andrew Burt is an executive business manager of the Digital Healthcare Business Unit at Analog Devices. He focuses on business development for the company’s healthcare products that include sensors, AFEs, optical modules, and algorithms. Andrew also helps define new sensors that will be part of future wellness and disease management solutions. He studied electrical and electronics engineering at Oxford Brookes University. He can be reached at andrew.burt@analog.com.
















