Magnesium batteries offer promise for safely powering modern life – unlike traditional lithium ion batteries, they are not flammable or subject to exploding – but their ability to store energy has been limited.
Researchers reported Aug. 24 in the journal Nature Communications the discovery of a new design for the battery cathode, drastically increasing the storage capacity and upending conventional wisdom that the magnesium-chloride bond must be broken before inserting magnesium into the host.
“We are combining a nanostructured cathode and a new understanding of the magnesium electrolyte,” said Yan Yao, associate professor of electrical and computer engineering at the University of Houston and lead author on the paper. “That’s new.”
The work was first conceived by Yao and postdoctoral fellow Hyun Deog Yoo in 2014; the project spanned several years and involved scientists from three universities and three national laboratories, working both experimentally and theoretically.
“Magnesium ion is known to be hard to insert into a host,” said Yoo, first author on the paper. “First of all, it is very difficult to break magnesium-chloride bonds. More than that, magnesium ions produced in that way move extremely slowly in the host. That altogether lowers the battery’s efficiency.”
The new battery stores energy by inserting magnesium monochloride into a host, such as titanium disulfide. By retaining the magnesium-chloride bond, Yao said, the cathode demonstrated much faster diffusion than traditional magnesium versions.
The researchers report the new battery has storage capacity of 400 mAh/g, compared with 100 mAh/g for earlier magnesium batteries. Commercial lithium ion batteries have a cathode capacity of about 200 mAh/g, said Yao, who is also a principal investigator with the Texas Center for Superconductivity at UH.
Voltage of the new battery remains low at about one volt. That compares to three to four volts for lithium batteries.
The high voltage, coupled with their high energy density, has made lithium ion batteries the standard. But lithium is expensive and can develop breaches in its internal structure, a condition known as dendrite growths, which can cause the batteries to catch fire. As an earth-abundant resource, magnesium is cheaper and does not form dendrites.
Until now, however, it has been held back by the need for a better cathode – the electrode from which the current flows – and more efficient electrolytes, the medium through which the ionic charge flows between cathode and anode.
This work suggests a solution.
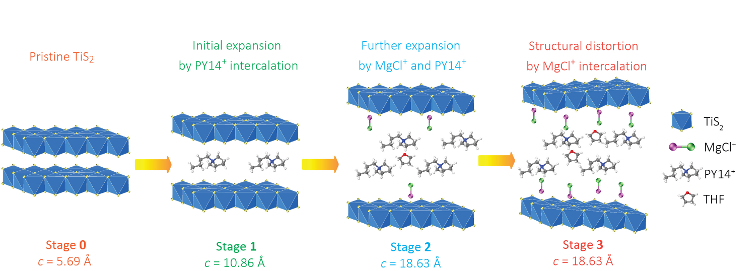
The key, Yoo said, is to expand the titanium disulfide to allow magnesium chloride to be inserted – a four-step process called intercalation – rather than breaking the magnesium-chloride bonds and inserting the magnesium alone. Retaining the magnesium-chloride bond doubled the charge the cathode could store.
The magnesium monochloride molecules are too large to be inserted into the titanium disulfide using conventional methods. Building upon their earlier work, the researchers created an open nanostructure by expanding the gaps in the titanium disulfide by 300 percent, using organic “pillars.”
The opening still was small, increased from 0.57 nanometers to 1.8 nanometers, but Yao said that allowed for the magnesium chloride to be inserted.
“Combined theoretical modeling, spectroscopic analysis, and electrochemical study reveal fast diffusion kinetics of magnesium monochloride cations without scission of magnesium chloride bond,” the researchers wrote. “… The large capacity accompanies excellent rate and cycling performances even at room temperature, opening up possibilities for a variety of effective intercalation hosts for multivalent-ion batteries.”
“We hope this is a general strategy,” Yoo said. “Inserting various polyatomic ions in higher voltage hosts, we eventually aim to create higher-energy batteries at a lower price, especially for electric vehicles.”
In addition to Yao and Yoo, authors on the paper include Yanliang Liang, Hui Dong, Yifei Li, Qiang Ru, Yan Jing and Qinyou An, all of UH; Junhao Lin and Sokrates T. Pantelides of Vanderbilt University and the Oak Ridge National Laboratory; Wu Zhou of Oak Ridge National Laboratory; Hua Wang and Xiaofeng Qian of Texas A&M University; Yisheng Liu and Jinghua Guo of Lawrence Berkeley National Laboratory; Lu Ma, Tianpin Wu and Jun Lu of Argonne National Laboratory.
By: Jeannie Kever